Answer:
The correct answer is option 'E': None of the above.
Step-by-step explanation:
Given that pH of the solution is 4.1537
From the basic relation of acids and bases we know that
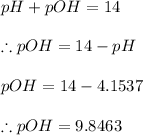
From the definition of pOH we have
![pOH=-log[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/9kreltumcuwfp30nq68lrhe5xxajx0hoy5.png)
where
[OH] is the concentration of hydroxide ions in moles/Liter (M)
Applying values and subsequently solving we get
![9.8463=-log[OH^(-)]\\\\\therefore [OH^(-)]=antilog(-9.8463)\\\\\therefore [OH^(-)]=10^(-9.8463)M](https://img.qammunity.org/2020/formulas/chemistry/college/oaiix6f3ls4ndkeyamyv0vimiiybrbd5bo.png)
![\therefore [OH^(-)]=14.246* 10^(-11)}M](https://img.qammunity.org/2020/formulas/chemistry/college/p5dmje00gkeufhvwqjmfxayxd92ric2i8j.png)