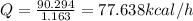Answer:
Resulting heat generation, Q = 77.638 kcal/h
Given:
Initial heat generation of the sphere,
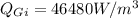
Maximum temperature,
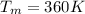
Radius of the sphere, r = 0.1 m
Ambient air temperature,
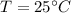 = 298 K
= 298 K
Solution:
Now, maximum heat generation,
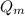 is given by:
is given by:
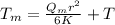 (1)
(1)
where
K = Thermal conductivity of water at
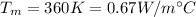
Now, using eqn (1):
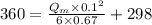
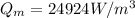
max. heat generation at maintained max. temperature of 360 K is 24924
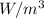
For excess heat generation, Q:
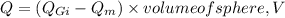
where
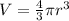
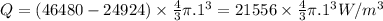
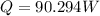
Now, 1 kcal/h = 1.163 W
Therefore,