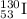Answer: The atomic symbol of the given element is
Step-by-step explanation:
The general isotopic representation of an element is given as:
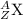
where,
Z represents the atomic number of the element
A represents the mass number of the element
X represents the symbol of an element
For the given isotope: 130-iodine
Mass number = 130
Atomic number = 53
Hence, the atomic symbol of the given element is