Answer:

Step-by-step explanation:
For n-=1 state hydrogen energy level is split into three componets in the presence of external magnetic field. The energies are,
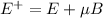 ,
,
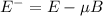 ,
,
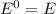
Here, E is the energy in the absence of electric field.
And
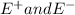 are the highest and the lowest energies.
are the highest and the lowest energies.
The difference of these energies
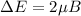
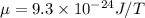 is known as Bohr's magneton.
is known as Bohr's magneton.
B=2.5 T,
Therefore,
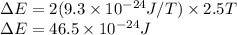
Now,
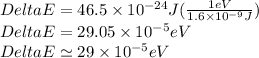
Therefore, the energy difference between highest and lowest energy levels in presence of magnetic field is
