Answer : The value of 'K' equilibrium constant is, 0.489
Solution : Given,
Volume of
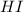 =
=
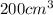
Volume of
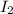 at equilibrium =
at equilibrium =

Volume of gas for 1 mole of gas =
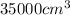
First we have to calculate the moles of
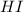 and
and
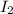 .
.
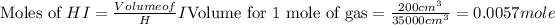
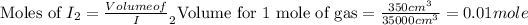
Now we have to calculate the value of equilibrium constant.
The given equilibrium reaction is,
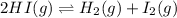
Initially moles 0.0057 0 0
At equilibrium (0.0057-2x) x x
The expression of
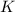 will be,
will be,
![K=([H_2][I_2])/([HI]^2)](https://img.qammunity.org/2020/formulas/physics/college/mvp695ce2aewg6kr3ef9wqqy20sitix14t.png)
Let the total volume be 'V'.
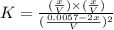
As per question,
Moles of
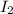 at equilibrium = x = 0.01
at equilibrium = x = 0.01
Now put the values of 'x' in the above expression, we get:
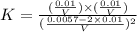
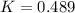
Therefore, the value of 'K' equilibrium constant is, 0.489