Answer:
W=3.327 KJ
Step-by-step explanation:
Given that
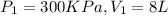
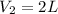
Temperature remains constant it means that this is isothermal process
For isothermal process
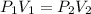
We know that work for isothermal process
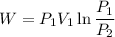
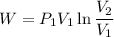
Now by putting the values
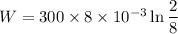
W=-3.327 KJ
Negative sign indicates is process is compression
W=3.327 KJ
So our option C is right.