Answer:
0.001970 moles of sodium oxalate are present in the flask.
Step-by-step explanation:
Mass of sodium oxalate = m = 0.2640 g
Molar mass of sodium oxalate =
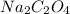 = 134 g/mol
= 134 g/mol
Moles of compound:n
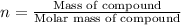
Number of moles of sodium oxalate:
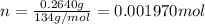
0.001970 moles of sodium oxalate are present in the flask.