Answer:
2.55 eV is absorbed.
Step-by-step explanation:
The energy of the nth state of hydrogen atom is,
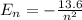
Now energy for the second state is,
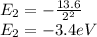
Now energy for the fourth state is,
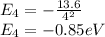
Now energy required to go from 2nd state to 4th state is,
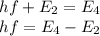
Therefore,
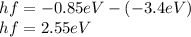
Therefore, 2.55 eV energy is absorbed by the hydrogen atom to from n=2 state to n=4 state.