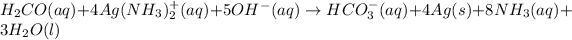Step-by-step explanation:
(a) The given reaction equation is as follows.
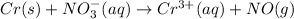 (acidic)
(acidic)
So, here the reduction and oxidation-half reactions will be as follows.
Oxidation-half reaction:
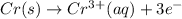
Reduction-half-reaction:
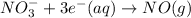
As total charge present on reactant side is -1 and total charge present on product side is +3. And, since it is present in aqueous medium. Hence, we will balance the charge for this reaction equation as follows.
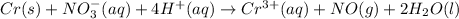 (acidic)
(acidic)
(b) The given reaction equation is as follows.
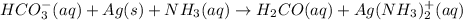 (basic)
(basic)
So, here the reduction and oxidation-half reactions will be as follows.
Reduction-half reaction:
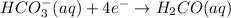
Oxidation-half reaction:
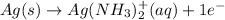
Hence, to balance the number of electrons in this equation we multiply it by 4 as follows.

Therefore, balancing the whole reaction equation in the basic medium as follows.