Answer :
(1) The pH of the solution after addition of 10.0 mL acid is, 4.19
(2) The pH of the solution after addition of 20.0 mL acid is, 10.77
Explanation :
(1) To calculate the pH of the solution after addition of 10.0 mL of acid.
First we have to calculate the moles of
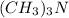 and
and
 .
.
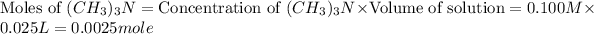
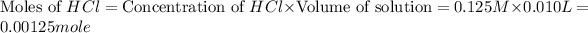
In this, HCl is limiting reactant because it is present in less amount.
The balanced chemical reaction is,
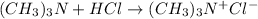
From the reaction we conclude that the mole ratio of
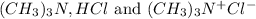 are 1 : 1 : 1.
are 1 : 1 : 1.
Moles of
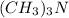 left = Initial moles of
left = Initial moles of
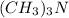 - Moles of
- Moles of
 added
added
Moles of
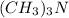 left = 0.0025 - 0.00125 = 0.00125 mole
left = 0.0025 - 0.00125 = 0.00125 mole
Moles of
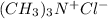 = Moles of HCl = 0.00125 mole
= Moles of HCl = 0.00125 mole
Now we have to calculate the
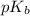
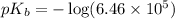
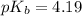
Now we have to calculate the pOH by using Henderson-Hasselbalch equation.
![pOH=pK_b+\log ([(CH_3)_3N^+Cl^-])/([(CH_3)_3N])](https://img.qammunity.org/2020/formulas/chemistry/college/gntqwdyy33ild0y1ntrgcq2dznf8dn31yg.png)
Total volume of solution = 25 + 10 = 35 mL = 0.035 L
Concentration of
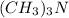 =
=
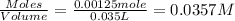
Concentration of
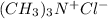 =
=
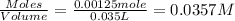
Now put all the given values in this expression, we get:
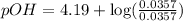
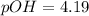
Now we have to calculate the pH of the solution.
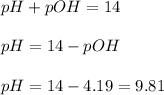
The pH of the solution after addition of 10.0 mL acid is, 9.81
(2) To calculate the pH of the solution after addition of 20.0 mL of acid.
First we have to calculate the moles of
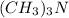 and
and
 .
.
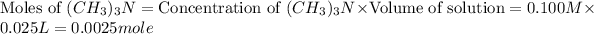
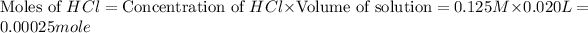
In this, HCl is limiting reactant because it is present in less amount.
The balanced chemical reaction is,
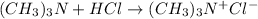
From the reaction we conclude that the mole ratio of
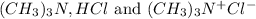 are 1 : 1 : 1.
are 1 : 1 : 1.
Moles of
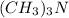 left = Initial moles of
left = Initial moles of
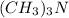 - Moles of
- Moles of
 added
added
Moles of
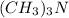 left = 0.0025 - 0.00025 = 0.00225 mole
left = 0.0025 - 0.00025 = 0.00225 mole
Moles of
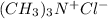 = MOles of HCl = 0.00025 mole
= MOles of HCl = 0.00025 mole
Now we have to calculate the pOH by using Henderson-Hasselbalch equation.
![pOH=pK_b+\log ([(CH_3)_3N^+Cl^-])/([(CH_3)_3N])](https://img.qammunity.org/2020/formulas/chemistry/college/gntqwdyy33ild0y1ntrgcq2dznf8dn31yg.png)
Total volume of solution = 25 + 20 = 45 mL = 0.045 L
Concentration of
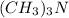 =
=
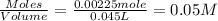
Concentration of
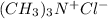 =
=
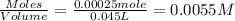
Now put all the given values in this expression, we get:
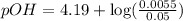
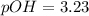
Now we have to calculate the pH of the solution.
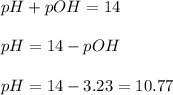
The pH of the solution after addition of 20.0 mL acid is, 10.77