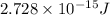Answer : The energy released by the decay of 3 grams of 'Th' is
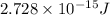
Explanation :
First we have to calculate the mass defect
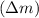 .
.
The balanced reaction is,
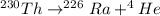
Mass defect = Sum of mass of product - sum of mass of reactants
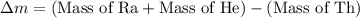
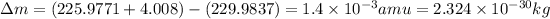
conversion used :
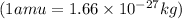
Now we have to calculate the energy released.
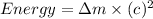
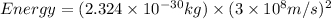
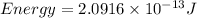
The energy released is
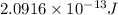
Now we have to calculate the energy released by the decay of 3 grams of 'Th'.
As, 230 grams of Th release energy =
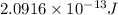
So, 3 grams of Th release energy =
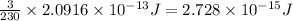
Therefore, the energy released by the decay of 3 grams of 'Th' is