Answer:
sp hybrid orbitals
Step-by-step explanation:
Acetylene molecule is linear in shape which means that all the four atoms lie in the straight line.
Carbon-carbon triple bond is 1.20Å long only. In acetylene, both the carbons are sp-hybridized which means that s orbital overlaps with one p orbital only. 2s orbital in sp-hybridized carbon combines with
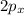 orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon.
orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon.
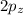 and
and
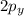 orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.
orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.