Answer:
No. of unpaired electrons in S = 2
Step-by-step explanation:
Atomic number of Sulfur = 16
Electronic configuration of S:
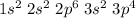
1s2, 2s2, 2p6 and 2s2 are totally filled. only 3p^4 is partially filled. p sub shell has three orbital. As per Hund's rule electrons in the orbital of sub shell fill in such a way that orbitals are first singly occupied and after that pairing occurs.
therefore, no. of unpaired electrons in S = 2