Step-by-step explanation:
Ammonia (NH₃)
Valence electrons of nitrogen = 5
Valence electrons of hydrogen = 1
The total number of the valence electrons = 5 + 3(1) = 8
The Lewis structure is drawn in such a way that the octet of each atom and duet for the hydrogen in the molecule is complete. So,
The Lewis structure is shown in the image below.
The molecular geometry is trigonal pyramidal as the central atom has one lone pair and the molecule is of the type
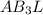 which corresponds to trigonal pyramidal geometry.
which corresponds to trigonal pyramidal geometry.