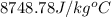Answer : The specific heat of unknown sample is,
Explanation :
In this problem we assumed that heat given by the hot body is equal to the heat taken by the cold body.
![q_1=-[q_2+q_3]](https://img.qammunity.org/2020/formulas/physics/college/qbxzm2m9ad9to73gicypr38dnhurr57hqw.png)
![m_1* c_1* (T_f-T_1)=-[m_2* c_2* (T_f-T_2)+m_3* c_3* (T_f-T_2)]](https://img.qammunity.org/2020/formulas/physics/college/7m15bsdmbe57mge99qpm4ryfd7dq0n6e9z.png)
where,
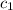 = specific heat of unknown sample = ?
= specific heat of unknown sample = ?
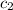 = specific heat of water =
= specific heat of water =
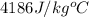
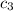 = specific heat of copper =
= specific heat of copper =
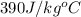
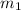 = mass of unknown sample = 72.0 g = 0.072 kg
= mass of unknown sample = 72.0 g = 0.072 kg
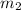 = mass of water = 203 g = 0.203 kg
= mass of water = 203 g = 0.203 kg
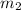 = mass of copper = 187 g = 0.187 kg
= mass of copper = 187 g = 0.187 kg
 = final temperature of calorimeter =
= final temperature of calorimeter =
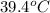
 = initial temperature of unknown sample =
= initial temperature of unknown sample =
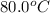
 = initial temperature of water and copper =
= initial temperature of water and copper =
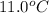
Now put all the given values in the above formula, we get
![0.072kg* c_1* (39.4-80.0)^oC=-[(0.203kg* 4186J/kg^oC* (39.4-11.0)^oC)+(0.187kg* 390J/kg^oC* (39.4-11.0)^oC)]](https://img.qammunity.org/2020/formulas/physics/college/yll7qucxl4jolewt65ptre5v4rot495toz.png)
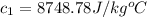
Therefore, the specific heat of unknown sample is,