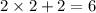Answer:
a). Coordination no. of
![[Ni(en)_2(NH_3)_2]^2+](https://img.qammunity.org/2020/formulas/chemistry/college/d64prarjgsx8imb5bdu46yhgctesy4vizq.png) = 6
= 6
b). Coordination no. of
![[Fe(en)(ox)Cl_2]^2-](https://img.qammunity.org/2020/formulas/chemistry/college/jvbxu1ttlkennc436n9jpu5t4jhjzbsfqs.png) = 6
= 6
Step-by-step explanation:
Coordination number is defined as number of donar atoms bonded to the central atom of the complex ion.
a). Coordination no. of
![[Ni(en)_2(NH_3)_2]^2+](https://img.qammunity.org/2020/formulas/chemistry/college/d64prarjgsx8imb5bdu46yhgctesy4vizq.png) = 6
= 6
en or ethylenediamine is a bidentate ligand.
In bidentate ligand, two atoms directly bonded to the central atom.
NH3 is a unidentate ligand.
So, coordination no.= No. of bidentate ligand x 2 + No. of unidentate ligand
=
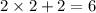
b). Coordination no. of
![[Fe(en)(ox)Cl_2]^2-](https://img.qammunity.org/2020/formulas/chemistry/college/jvbxu1ttlkennc436n9jpu5t4jhjzbsfqs.png) = 6
= 6
Ethylenediamine (en) is a bidentate ligand.
oxalate ion (ox) is also a bidentate ligand.
Cl is a unidentate ligand
So, coordination no.= No. of bidentate ligand x 2 + No. of unidentate ligand
=