Answer:
0.045 M/s
Step-by-step explanation:
Given:
Initial concentration of A = 0.625 M
Final concentration of A = 0.100 M
Total time taken for the change of concentration = 11.6 seconds
The average rate of reaction is calculated as:
=
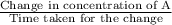
on substituting the respective values, we get
=
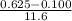
or
The average rate of reaction = 0.045 M/s