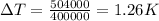Answer:
(A) 140 j/sec (b) 1.26 K
Step-by-step explanation:
We have given the heat heat flowing into the refrigerator = 40 J/sec
Work done = 40 W
(a) So the heat discharged from the refrigerator
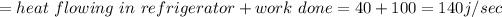
(b) Total heat absorbed =140 j/sec
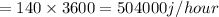
Let the temperature be

Heat absorbed per hour =504000
![[tex]=400* 10^3* \Delta T](https://img.qammunity.org/2020/formulas/physics/college/qzkxj0lp9qmrdu48sc71ugpb6mr0zaw0vs.png)
So