Answer: Aluminium
Step-by-step explanation:
Given reduction potentials:
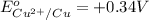
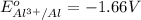

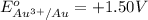
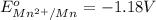
The elements with negative reduction potential will lose electrons undergo oxidation and thus act as good reducing agents.The elements with positive reduction potential will gain electrons undergo reduction and thus acts as good oxidizing agents.
Looking at the given standard reduction potentials, element aluminium can most easily lose electrons, get mostly easily oxidized and hence act as strongest reducing agent.