Answer: When bleach is mixed with water, it produces hypochlorous acid.
Step-by-step explanation:
The chemical name for bleach is sodium hypochlorite. When this compound is reacted with water, it produces hypochlorous acid and sodium hydroxide.
The chemical equation for the reaction of sodium hypochlorite and water follows:
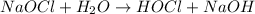
By Stoichiometry of the reaction:
1 mole of sodium hypochlorite reacts with 1 mole of water to produce 1 mole of hypochlorous acid and 1 mole of sodium hydroxide.
Hence, when bleach is mixed with water, it produces hypochlorous acid.