Step-by-step explanation:
The given data is as follows.
Density of vinegar = 1.0 g/ml
Specific heat capacity = 4.25
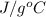
 =
=
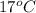 , and
, and
 =
=
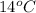
Relation between enthalpy and specific heat is as follows.
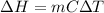
Hence, putting the values into the above formula as follows.
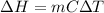
=
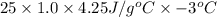 (as density =
(as density =
 )
)
= - 315 J
Thus, we can conclude that the enthalpy of reaction is -315 J.
As the value is negative so, it means that heat is releasing. Hence, the reaction is exothermic in nature.