Answer: Option (a) is the correct answer.
Step-by-step explanation:
As heat is absorbed by the reactant in this chemical reaction. Hence, it is an endothermic reaction.
According to Le Chaltelier's principle, any change in an equilibrium reaction will shift the equilibrium in a direction that will oppose the change.
So, when we increase the concentration of reactant here, that is,
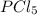 then the equilibrium will shift in the forward direction.
then the equilibrium will shift in the forward direction.
This means that there will be increase in concentration of
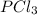 .
.
Thus, we can conclude that adding
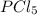 will cause an increase in the concentration of
will cause an increase in the concentration of
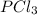 in the given reaction.
in the given reaction.