Answer:
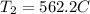
ΔU =195 KJ
W=0 KJ
Step-by-step explanation:
Given that
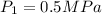
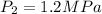
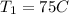
Mass of air m=3 kg
heat absorb by air =195 KJ
Cv=0.741 KJ/kgK
If we assume that air is as ideal gas so
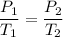
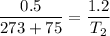
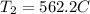
Given that container is rigid it means that volume of system is constant so W=0
From first law of thermodynamics
Q=ΔU + W
⇒Q= ΔU (W=0)
So ΔU =195 KJ
And work done will be zero because because it is a constant volume process.