Answer:
Final temperature of the gas = -146.63 °C
Step-by-step explanation:
At constant pressure, volume and temperature of the gases are related as:
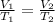
Where,
V1 = Initial volume = 1.00 L
V2 = Final volume = 2.40 L
T1 = Initial temperature = 30.5 °C = 30.5 + 273.15 = 303.65 K
Now, substitute the values in the above equation,
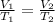
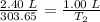
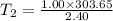
T2 = 126.52 K
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
T( °C) = T(K) - 273.15
= 126.52 - 273.15 = -146.63 °C