Answer:
Liming reagent in the given reaction is oxygen.
Step-by-step explanation:
Mass of octane = 12.85 g
Mass of oxygen = 7.46 g
Molar mass of octane = 114.23 g/mol
Molar mass of oxygen = 32
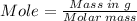
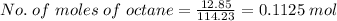
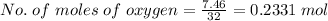
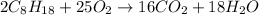
According to the reaction, 2 moles of octane requires 25 moles of O2
so, 0.1125 moles of octane requires
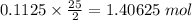 of oxygen.
of oxygen.
but only 0.2331 mol of oxygen is present.
Hence, oxygen is the limiting reagent