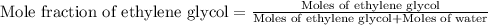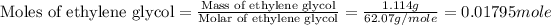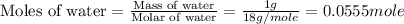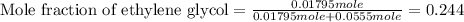Answer :
(a) The volume percent is, 50.63 %
(b) The mass percent is, 52.69 %
(c) Molarity is, 9.087 mole/L
(d) Molality is, 17.947 mole/L
(e) Moles fraction of ethylene glycol is, 0.244
Explanation : Given,
Density of ethylene glycol = 1.114 g/mL
Molar mass of ethylene glycol = 62.07 g/mole
Density of water = 1.00 g/mL
Density of solution or mixture = 1.070 g/mL
According to the question, the mixture is made by mixing equal volumes of ethylene glycol and water.
Suppose the volume of each component in the mixture is, 1 mL
First we have to calculate the mass of ethylene glycol.
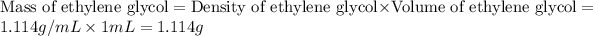
Now we have to calculate the mass of water.
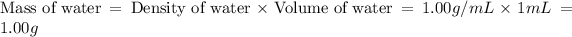
Now we have to calculate the mass of solution.
Mass of solution = Mass of ethylene glycol + Mass of water
Mass of solution = 1.114 + 1.00 = 2.114 g
Now we have to calculate the volume of solution.
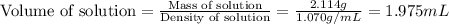
(a) Now we have to calculate the volume percent.
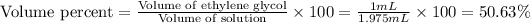
(b) Now we have to calculate the mass percent.
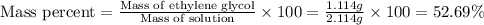
(c) Now we have to calculate the molarity.
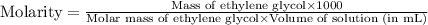
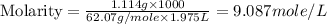
(d) Now we have to calculate the molality.
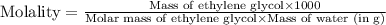
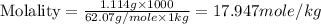
(e) Now we have to calculate the mole fraction of ethylene glycol.