Explanation :
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
Or we can say that, conjugate acid is proton donor and conjugate base is proton acceptor.
The equilibrium reaction will be,
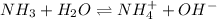
In this reaction,
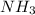 and
and
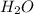 are base and acid and
are base and acid and
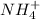 and
and
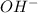 are conjugate acid and conjugate base respectively.
are conjugate acid and conjugate base respectively.
Ammonia is a weak base because it accept proton from the water and gives fewer hydroxide ions.