Answer:
Final temperature of the sample is 92.88 degrees Celsius.
Step-by-step explanation:
It is given that,
Mass of the sample of copper, m = 68 g = 0.068 kg
Initial temperature,
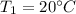
Energy added to the copper, Q = 1918 J
Specific heat of the copper,
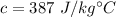
We need to find the final temperature of the copper sample. The heat added is given by :
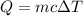
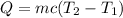 , T₂ is the final temperature
, T₂ is the final temperature
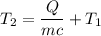
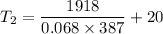
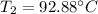
So, the final temperature of the copper sample is 92.88 degrees Celsius. Hence, this is the required solution.