Answer:
Solid become more soluble
Step-by-step explanation:
The given reaction:
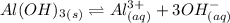
On the addition of HCl to the equilibrium, the equilibrium will shift towards right which means more dissociation of aluminum oxide. This is because the furnished hydroxide ions from aluminum oxide on dissolution now binds with the protons furnished by the acid to form water and thus there become less concentration of the hydroxide ions on the right side and to nullify this effect, the equilibrium will shift in right direction.
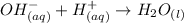
Solubility product is defined as the product of the concentration of the ions of the solid. More the solubility product more is the solubility.
Thus, on addition of the acid, the solid become more soluble.