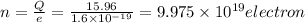Answer:
(a) 15.96 C (B)47.88 J (C)
Step-by-step explanation:
We have given current i =0.133 A
Voltage of the battery V =3 volt
Time t=2 minute=2×60=120 sec
(a) We have to find the charge
charge Q = current ×time
So
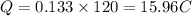
(B) The energy supplied by the battery
Energy = charge ×potential difference
So
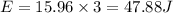
(C) We know that charge on one electron

So number of electron