Answer: The reaction is non spontaneous at
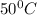
Step-by-step explanation:
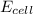 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous
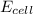 = -ve, reaction is non spontaneous
= -ve, reaction is non spontaneous
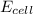 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
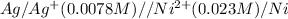
Using Nernst equation :
![E_(cell)=E^o_(cell)-(2.303RT)/(nF)\log ([Ag^(+)])/([Ni^(2+)]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/q4fhe5jy7rifom4nkupafv8cgbo280ak6a.png)
where,
R = gas constant =
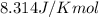
T= temperature in kelvin =
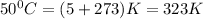
n = number of electrons in oxidation-reduction reaction = 2
F= Faraday's constant = 96500 C
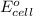 = standard electrode potential =
= standard electrode potential =
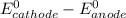
Where both
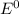 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Ag^(+)/Ag])= +0.80V](https://img.qammunity.org/2020/formulas/chemistry/college/w4dleiocetp3bkk5ha0zk897u2sqgrj2yo.png)
![E^0_([Ni^(2+)/Ni])= -0.23V](https://img.qammunity.org/2020/formulas/chemistry/college/ipsakxi13l2e4uykturuffzb1ht56u9xb7.png)
![E^0=E^0_([Ni^(2+)/Ni])- E^0_([Ag^(+)/Ag])](https://img.qammunity.org/2020/formulas/chemistry/college/qg45w3wxfbynl78eeuq92l6ii1c7ys20po.png)
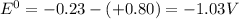
![E_(cell)=-1.03 - (2.303* 8.314* 323)/(2* 96500)\log ([0.0078])/([0.023]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/7hmbvf5t6bf4cu5d4jz0ssgjyxgz2ihjcu.png)
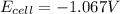
Thus as
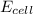 is negative , the reaction is no spontaneous.
is negative , the reaction is no spontaneous.