Answer:
(a) A strong acid
Step-by-step explanation:
We have given the pH of the solution is 2.46
pH=2.46
So the concentration of
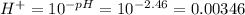
solution having H+ concentration more than
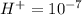 is acidic
is acidic
Since in the given solution, H+ concentration is 0.00346 M which is more than 10^{-7}[/tex] so this is an acidic solution
Note-The concentration of
 decide the behavior of the solution that is, it is acidic or basic
decide the behavior of the solution that is, it is acidic or basic