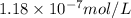Answer: The solubility of
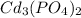 in water is
in water is
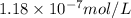
Step-by-step explanation:
The balanced equilibrium reaction for the ionization of cadmium phosphate follows:
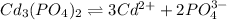
3s 2s
The expression for solubility constant for this reaction will be:
![K_(sp)=[Cd^(2+)]^3[PO_4^(3-)]^2](https://img.qammunity.org/2020/formulas/chemistry/college/wa9ayv3hbchlb2xvve92idajw0nq1dypv3.png)
We are given:
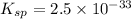
Putting values in above equation, we get:
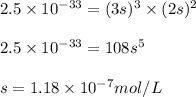
Hence, the solubility of
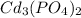 in water is
in water is