Answer: The molecular equation is written below.
Step-by-step explanation:
Molecular equation is defined as the equation in which ionic compounds are expressed as compounds instead of constituent ions.
The balanced molecular equation for the reaction of zinc and hydrochloric acid follows:
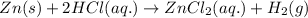
By Stoichiometry of the reaction:
1 mole of zinc metal reacts with 2 moles of hydrochloric acid to produce 1 mole of zinc chloride and 1 mole of hydrogen gas.
Hence, the molecular equation is written above.