Answer: 1.96 grams
Step-by-step explanation:
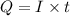
where Q= quantity of electricity in coloumbs
I = current in amperes = 0.385 A
t= time in seconds = 1.259 hours = 4532.4 sec (1hour =3600 s)
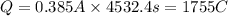
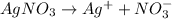
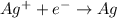
96500C of electricity deposits 1 mole of Ag
1755 C of electricity deposits =
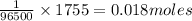 of Ag
of Ag
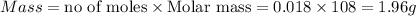
Thus 1.96 g of silver can be electroplated when 0.385 amps are used for 1.259 hours using a solution of silver nitrate.