Answer:
There are approximately 475 umol of chlorogenic acid in the sample.
Step-by-step explanation:
The first step is indentifying the chlorogenic acid structure. As it can be seen in the figure attached, this molecule is a carboxylic acid containing just one carboxyl group. This means, that chlorogenic acid is a monoprotic acid and it is only able to donate one proton per molecule or one mol of protons per one mol of molecules.
The second step is to balance the titration equation. Considering that sodium hydroxide will generate one mol of hydroxyl ions per mol of salt, we can simplify the equation:
H⁺ + OH ⁻ → H₂O
Therefore, we now know that for each mol of NaOH consumed 1 mol of chlorogenic acid is titrated.
Thus, the last step is calculation the amount of NaOH consumed during the tritation. We can use the following equation:
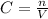
In which C is the concentration, n the amount of moles and V the volume.
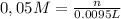
The result is that n = 475 umol.