Answer:
The mass of the precipitate that AgCl is 3.5803 g.
Step-by-step explanation:
a) To calculate the molarity of solution, we use the equation:
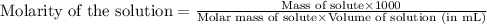
We are given:
Mass of solute (NaCl) = 1.46 g
Molar mass of sulfuric acid = 58.5 g/mol
Volume of solution =
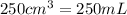
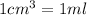
Putting values in above equation, we get:
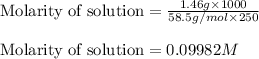
0.09982 M is the concentration of the sodium chloride solution.
b)
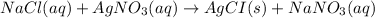
Moles of NaCl =
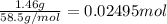
according to reaction 1 mol of NaCl gives 1 mol of AgCl.
Then 0.02495 moles of NaCl will give:
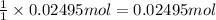 of AgCl
of AgCl
Mass of 0.02495 moles of AgCl:
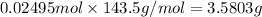
The mass of the precipitate that AgCl is 3.5803 g.