Answer: cations
Step-by-step explanation:
Metals are defined as the elements which loose electrons to attain stable electronic configuration. If an atom looses electrons, it leads to the formation of positive ions known as cations.
For Example: Sodium looses 1 electron to form
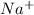 ions.
ions.
Non-metals are defined as the elements which gain electrons to attain stable electronic configuration. If an atom gains electrons, it leads to the formation of negative ions known as anions.
For Example: Fluorine gains 1 electron to form
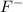 ions.
ions.