Answer:
0.096 M
Step-by-step explanation:
Given:
50.00 ml of 0.0565 M AgNO₃ was added to 25.00 ml of a potassium iodide solution
8.32 ml of 0.0510 M KSCN solution to precipitate the unreacted silver ions
Now,
The moles of AgNO₃ added = Volume of solution × Concentration
or
The moles of AgNO₃ added = 0.05 × 0.0565 = 0.002825 moles
and
The number of moles of KSCN reacted
= Volume × Concentration
= 0.00832 × 0.0510
= 0.00042432 moles
Now,
The number of moles of AgNO₃ that reacted with KI
= The moles of AgNO₃ added - The number of moles of KSCN reacted
= 0.002825 - 0.00042432
= 0.00240068
Therefore,
The concentration of the original KI solution =
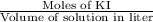
or
The concentration of the original KI solution =
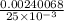
or
The concentration of the original KI solution = 0.096 M