Answer:
T= 25.0061 °C
Step-by-step explanation:
Given that
For iron ball:
d=5 cm
We know that for iron
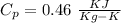
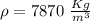
So the mass of iron
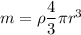
Now by putting the values
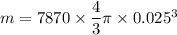
m=0.51 kg
Foe water:
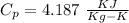
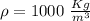
m=1000 x 0.5 kg
m=500 kg
So
heat loss by iron ball = heat gain by water
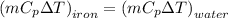
Lets take T is the final temperature
0.51 x 0.46 (80 -T) = 500 x 4.187 x (T-25)
T= 25.0061 °C