Answer:
0.0793 mol.
Step-by-step explanation:
Refer to a modern periodic table for relative atomic mass data:
- Cu: 63.546;
- N: 14.007;
- O: 15.999.
Formula mass of copper(II) nitrate,
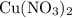 :
:
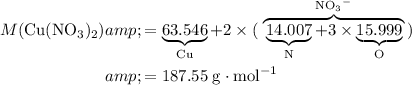 .
.
Number of moles of copper(II) nitrate produced:
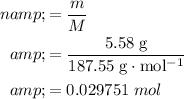 .
.
The ratio between the coefficient of
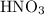 and that of
and that of
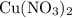 in the balanced equation is:
in the balanced equation is:
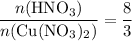 .
.
In other words,
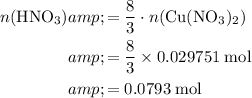 .
.