Answer:
The kinetic energy would also be increased by 138 times.
Step-by-step explanation:
Rest mass of an electron
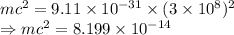
Convert to MeV
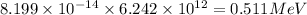
Total energy of the electron = 138×mc² =70.518 MeV
Kinetic energy of an electron
E = mc²
According to the question
E = 138×0.511 MeV = 70.518 MeV
So, the kinetic energy would also be increased by 138 times.