Answer:
7 grams of iron is produced from the reduction of 10.0 g of iron (III) oxide.
Step-by-step explanation:
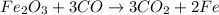
Moles of iron (III) oxide :
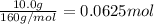
According to reaction, 1 mol of iron(III) oxide gives 2 moles of iron metal.
Then 0.0625 moles of iron(III) oxide will give:
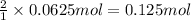 of iron metal.
of iron metal.
Mass of 0.125 moles of iron metal.
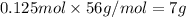
7 grams of iron is produced from the reduction of 10.0 g of iron (III) oxide.