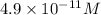Answer: The concentration of
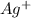 ion in the solution is
ion in the solution is
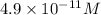
Step-by-step explanation:
The balanced equilibrium reaction for the ionization of calcium fluoride follows:
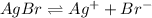
s 0.01
The expression for solubility constant for this reaction will be:
![K_(sp)=[Ag^(+)][Br^-]](https://img.qammunity.org/2020/formulas/chemistry/college/ljl7gvndjeua79krr43izzsz5nhd7bsg8r.png)
We are given:
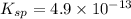
![[Br^-]=0.01M](https://img.qammunity.org/2020/formulas/chemistry/college/m29zc4nj5i1ey3926g1evr8hlgghc3zrmn.png)
Putting values in above equation, we get:
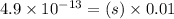
![[Ag^+]=4.9* 10^(-11)M](https://img.qammunity.org/2020/formulas/chemistry/college/ira5g3sqw99brtn6fuf5dmybgi6yej1ecv.png)
Hence, the concentration of
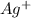 ion in the solution is
ion in the solution is