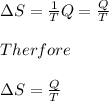Answer:
∆S=Q/T
Step-by-step explanation:
Given the general expression for entropy:
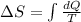
We can proceed to find the final expression.
As the temperature does not change, it can be removed from the integral, leaving:
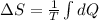
Now, we proceed to solve the integral to have the final answer: