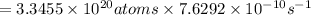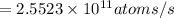Answer:
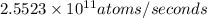 is the activity, measured in of a 50 mg sample of 90-Sr.
is the activity, measured in of a 50 mg sample of 90-Sr.
Step-by-step explanation:
Half life of the 90-Sr ,
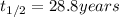
Activity coefficient of the 90-Sr =

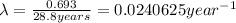
Mass of 90-Sr = 50 mg = 0.050 g
Molecular mass of 90-Sr = 90 g/mol
Moles of 90-Sr =
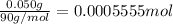
Number of atom in 0.0005555 moles of 90-Sr:
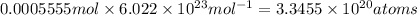
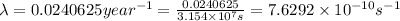
1 year =
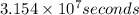
Activity measured in atoms per seconds:
= Number of atoms ×