Answer:
Answer B) 4.2x10^17
Step-by-step explanation:
To produce the reaction 3 using reaction 1 and 2 we need to invert the order of the first reaction the second in the same order, as it's shown:
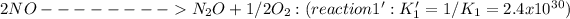
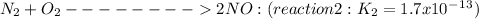
____________________________

Due to the inversion of the first equation, the equilibrium constant of the new reaction is K1'=1/K1.=2.4x10^30
Finally, the new equilibrium constant K3 is the product of the previous constants:
K3=K1'*K2=4.2x10^17