Answer:
(a). The change in the average kinetic energy per atom is
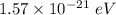 .
.
(b). The change in vertical position is 2413 m.
Step-by-step explanation:
Given that,
Mass = 40.0 u
The increased temperature from 286 K to 362 K.
(a). We need to calculate the change in the average kinetic energy per atom
Using formula of kinetic energy
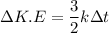
Put the value into the formula
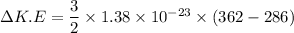

(b). The change in potential energy of the container due to change in the vertical position
We need to calculate the change in vertical position
Using formula of potential energy
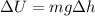
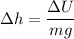
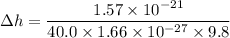
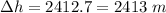
Hence, (a). The change in the average kinetic energy per atom is
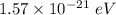 .
.
(b). The change in vertical position is 2413 m.