Answer:
4520 kJ
Step-by-step explanation:
To make a substance transition phases an energy must be applied, this is know as the latent heat.
Water has a latent heat of 40.7 KJ/mol to turn into vapor.
The molecule of water has a molecular weight of 18. This means that a mol of water weights 18 grams.
With this we can convert the latent heat to use grams instead of mols
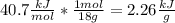
Now, the heat needed to vaporize water is simply:
Q = m * DHvap = 2000 g * 2.26 kJ/g = 4520 kJ