Step-by-step explanation:
The reaction equation will be as follows.
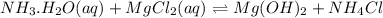
 of
of
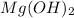 = 13 and
= 13 and
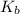 of
of
 =
=
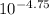
Hence, concentration of
 =
=
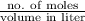
=
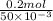
= 4 M
As,
![Mg(OH)_(2) \rightleftharpoons Mg^(2+) + 2[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/cjq5k8ku61l3vv4fjxlu0pxufrza4twxzw.png)
Hence,
![K_(sp) = [Mg^(2+)][OH^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/h7h5unlqdz0ncdr2k47aycs0aej3oa7qvs.png)
13 =
![4 * [OH^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/27n2yew12a03t2u2vtlgxyq8y62fslaol9.png)
![[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/ug5fh4frhjslztec5ab506a86sstd1ikl9.png) = 1.80276 M
= 1.80276 M
Also, ionization constant of water is given as follows.
![K_(w) = [H_(3)O^(+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/zdxko4etze6sidnrvefjwtszssv8u7zbbj.png) ......... (1)
......... (1)
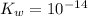
Now, put the value of
![[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/ug5fh4frhjslztec5ab506a86sstd1ikl9.png) in equation (1) as follows.
in equation (1) as follows.
![K_(w) = [H_(3)O^(+)][OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/zdxko4etze6sidnrvefjwtszssv8u7zbbj.png)
![[OH^(-)] = (K_(w))/([H_(3)O^(+)])](https://img.qammunity.org/2020/formulas/chemistry/college/trs5v8sie27ptmiiells1321ye31w0utfr.png)
=
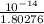
=
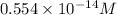
Also,
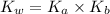 =
=
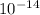
Therefore,
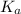 =
=
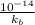
=
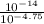
=
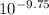
Since,
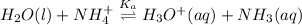
![K_(a) = ([H_(3)O^(+)[NH_(3)]])/([NH^(+)_(4)])](https://img.qammunity.org/2020/formulas/chemistry/college/5or0hr6i72v22kd83xjvezdf91j768wpzj.png) ....... (2)
....... (2)
Hence, calculate the value of
![[NH^(+)_(4)]](https://img.qammunity.org/2020/formulas/chemistry/college/ni0h2ml30iv7asz94egopc9kn40no43gkm.png) as follows.
as follows.
![[NH^(+)_(4)]](https://img.qammunity.org/2020/formulas/chemistry/college/ni0h2ml30iv7asz94egopc9kn40no43gkm.png) =
=
![([H_(3)O^(+)][NH_(3)])/(K_(a))](https://img.qammunity.org/2020/formulas/chemistry/college/2ucww7pgerfq313haonvm14dtgsdzgcsxj.png)
=
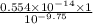
=
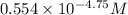
![[NH^(+)_(4)]](https://img.qammunity.org/2020/formulas/chemistry/college/ni0h2ml30iv7asz94egopc9kn40no43gkm.png) =
=
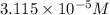
Thus, we can conclude that the minimum concentration of
![[NH^(+)_(4)]](https://img.qammunity.org/2020/formulas/chemistry/college/ni0h2ml30iv7asz94egopc9kn40no43gkm.png) that prevents the precipitation of
that prevents the precipitation of
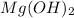 is
is
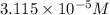 .
.